Tags
aquaculture, cytochalasin B, huître des quatre saisons, meiosis, polar body, polyploidy, tetraploid oysters, triploid oysters
Long ago were the halcyon days of seas, brimming with all kinds of fish and molluscs, and laissez-faire politics that shunned any form of regulation on fishing, best exemplified by the opinions of one of the intellectual giants of the 19th century, Thomas Huxley, an autodidactic natural scientist, who dubbed himself “Darwin’s bulldog” because of his staunch defence of the latter’s controversial ideas, and who fathered a long line of Huxley geniuses. In his opinion, the bounties of the oceans were considered inexhaustible and nature left to its own devices, in the true spirit of free trade and liberalism, was almost infinitely resilient and could adapt to any pressure imposed by man so that the idea of any threat of overfishing was totally rejected. To be fair to Huxley, towards the end of his life, his tone changed, as he became more convinced that management of oyster beds needed to be introduced and recognised the dangers inherent in certain practices. Apart from France, most countries failed to introduce any viable system of regulation until it was too late. Nowadays, the tragedy of all this naivety, on the one hand and greed, on the other, is only too apparent. Stocks have fallen dramatically and fishing has now become far more regulated. Aquaculture is seen as an economic and environmental necessity to safeguard the finite resources of the seas. However, not all aquaculture is sustainable, and in a recent book by Colin Nash, The History of Aquaculture (2011), a pile of evidence is amassed of the unsavoury involvement of the nuclear power industry and multinational chemical conglomerates like Union Carbide, Dow Chemical and Sun Oil in aquaculture during the 1960/70’s which had devastating consequences for the marine environment. Aquaculture was seen as a way to buy good publicity and acquire a brand as a caring company.
Right from the beginning, science has endeavoured to involve itself in aquaculture. One of its pioneers, known in France as le père de la pisciculture, Victor Coste (1807-1873) was originally professor of embryology, and was instrumental in spreading interest in the methods of artificial collection of wild spat from oysters. His was the age of the first hatcheries which were established to study and allow fish spawn in artificial environments. But science was generally slow to latch on. The first experimental hatcheries on a larger scale were started in the 1930’s, in Conwy, Wales (UK) under first Herbert Cole (1911-1984) and later Peter Walne (1926-1978) and in Milford, Connecticut (US) under Victor Loosanoff (1899-1987). But most of the work only got off the ground after the 2nd World War. Selective breeding and artificial rearing of oyster spat in hatcheries were seen as ways to compensate for the disappearance of wild stocks and unpredictability of spawning in colder climates by providing an almost limitless source of spat for cultivation. The first commercial oyster seed hatchery opened on the US west coast in 1967, but like most hatcheries was beset with various biological problems.
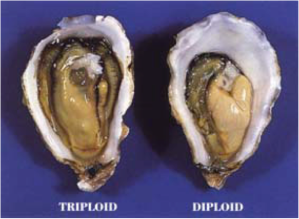
So this was the scene when a young and ambitious student set about trying to create a hybrid oyster, one which had never existed in nature. The narrative begins in a wooded, hilltop research centre, now the Ira C. Darling Marine Center, overlooking the Damariscotta River, on the Maine coast in North Eastern America, where in 1979 marine biologists at the University of Maine were working on methods to help improve the local shellfish industry. It was important to find ways to make fish grow more quickly in the colder waters, to overcome the problems of erratic spawning at such low temperatures and to make more money by producing shellfish for consumption the whole year round. The idea of growing brood stock in hatcheries was not new but producing a sterile oyster was, one that would be denied nature’s most basic function, reproduction, so that meat content, flavour and texture could be improved. Instead of utilising its sugar reserves of glucose and glycogen for gamete production, and reducing its meat content by as much as 70%, the sterile oyster, it was thought, could be freed to harness this energy for meat and shell growth, thus reducing the time to cultivate a marketable oyster. Another benefit in a faster growing oyster was that it could reach market size before being vulnerable to particular types of parasites like the one causing Dermo disease (Perkinsus marinus). In a word, the sterile triploid was going to be created because it made irrefutably marketing sense.
The Ira C. Darling Marine Center at the University of Maine
The story of the triploid oyster is a fascinating and to some extent frightening chapter in the history of aquaculture. It epitomises man’s desire to master and rise above the unpredictability of nature, but it also poses uncomfortable questions about the lengths to which man has gone in the pursuit to modify the ecology of nature. As Sir Maurice Yonge (1899-1986), a distinguished marine zoologist of his day, wrote in his Oysters on the future of oyster culture, “the more man interferes with nature the greater become the problems he creates (1960, 189).
Some elementary facts about genetic biology may be needed here. In the animal kingdom, nearly all species are diploid, that is, each of their somatic cells contains two sets of homologous chromosomes, one each from the male and female parent. Somatic cells give rise to the development of the individual body through the process of mitosis, in which cells divide through DNA replication, thus retaining their two sets of identical chromosomes. Germ cells, which are responsible for reproduction and formation of a new generation, are located in the gonads and develop into male and female gametes (i.e. sperm and egg). This process whereby germ cells recombine their genetic DNA molecules of homologous chromosomes (synapsis) and lose one of their sets of chromosomes and become haploid (a single set of chromosomes) progeny cells or gametes is called meiosis. As a couple of genetic biologists[1] so succinctly wrote, “the very essence of sex is meiotic recombination.” (We never learnt that at school!). Meiosis involves intricate phases of chromosomal separation, rearrangements and segregation before new haploid cells are formed, all within a relatively short period of time, although it is divided into two main stages, meiosis I and II. In each of these two stages, crucially so-called polar bodies are extruded (released) and serve as biological indicators of the development of meiosis, especially in the creation of triploid egg cells. However, the process of meiosis in many marine molluscs, including oysters is delayed and only completed after fertilisation, whereas in most other animals this process is achieved before fertilisation. It is this complicated and amazing process of meiosis that is manipulated, by inhibiting or blocking the release of the polar bodies either in meiosis I or meiosis II, in order to ensure that the egg retains its two sets of chromosomes. Normally, one set of chromosomes would be shed to make way for the set of chromosomes provided by the male sperm to secure the continuation of diploidy in the organism. If this manipulation succeeds, then the fertilised egg contains three sets of chromosomes, that is becomes a triploid cell, which then can undergo mitosis in the usual way. It was generally assumed that adult triploids were sterile since their three sets of homologous chromosomes could not successfully recombine during meiosis.
In humans and mammals generally, the condition of triploidy is always life-threatening, if not lethal, but in the non-vertebrate and plant world, there are many species, which exist in natural states of polyploidy (several sets of chromosomes). For instance, there are wild species of berries belonging to the genus of Vaccinium, like blueberries, cranberries and lingonberries that are polyploid (tetraploid and hexaploid), as well as diploid. There are even varieties of grapes that have been discovered to have this feature. Some common agricultural fruits, such as melons, bananas and oranges have also been manipulated into polyploids to grow bigger and more quickly.
Meanwhile back in Maine, research was geared to creating polyploid shellfish, and after a series of trial and error experiments, one technique, which had been used on clams as well as salmon and rainbow trout in Norway earlier in the early 1970’s, was selected with its fair share of serendipity. It involved the insertion of a toxic chemical, a mycotoxin, cytochalasin B, at a critical moment during meiosis into the newly fertilised egg to prevent the reduction of the two sets of the female chromosomes to one, so that it would end up with three sets (triploidy). Timing, duration and dosage levels were crucial and could in worst cases cause genetic abnormalities (aneuploidy) and high mortalities at various stages of larval development. The optimal point when the toxic chemical was inserted was during meiosis II, to inhibit the release of the second polar body and thus produce a triploid zygote (fertilised egg).

The development of chemically induced triploid zygotes during meiosis II
This laboratory technique of using cytochalasin B was gradually perfected and ushered in a new era in oyster cultivation, in which an artificial, supposedly sterile species, not genetically modified however, the triploid, could be used to produce a more meaty and juicy oyster more quickly, and even during the summer, “r-less” months. The young graduate student behind this work was Standish K. Allen Jnr, who together with his supervisor Herb Hidu and mentor Jon Stanley, is credited with the innovative research, conducted with the Eastern or Atlantic oyster, Crassostrea virginica, although he did not bother to get his “invention” patented. Their paper[2] in 1981 already mooted the idea of creating oysters with an even number of chromosome sets, like tetraploids (four sets), which then could synapse and be fertile. However, the local oyster farmers in Maine were too conservative then to embrace this new technology and the hatcheries that existed were small and more experimental than commercial.
So Allen jetted off in 1983 instead to the Northwest, eventually to complete his doctoral studies with a well-known biologist in the field, Kenneth Chew, in Seattle, where the oyster industry was far more commercialised, and ready at work on the Pacific oyster, Crassostrea gigas. Since this latter oyster generally was unable to spawn naturally in the colder Pacific water, well-established hatcheries had already begun to produce diploid oyster seed for cultivators to grow. He and another researcher, Sandra Downing, successfully applied the technique in 1985 to large batches of oysters in a commercial hatchery setting, whose owners wanted the process patented. The patent was in due course refused on the grounds that an earlier publication (in 1981) of the process meant that it was no longer original. The end result of the application in 1987, however, did create a historical precedent, as a landmark court case, since it was admitted for the first time ever that patents could be granted to new species of animals, genetically altered or modified by science. Suddenly, the door to the world of modern biotechnology was thrown open wide by this ruling.
Even so, health concerns about the carcinogen, cytochalasin B, were growing, because of its links with cancer and the FDA (the Food and Drug Administration) was debating whether to ban its use in commercial hatcheries. The two researchers decided to try another method to produce triploids by subjecting oyster eggs to hydrostatic pressure, and this time their patent application was accepted. Another method that was also used was subjecting the onsetting phase of meiosis to temperature extremes. An alternative to cytochalasin B has been the use of an enzyme inhibitor, 6-dimethylaminopurine (6-DMAP). However, the downsides of these four forms of induced triploidy was that they resulted in high mortalities of the oyster larvae in the hatcheries due to the severity of the treatment, that the success rate varied and that some triploid oysters were unstable enough to revert back into diploids as they grew or were able to spawn themselves, and so were not wholly sterile. There were other contradictions that triploids produced earlier in meiosis (so-called meiosis I) grew faster but were liable to higher mortalities than triploids produced later during meiosis II. But faster growth could also have been due to the fact that triploid cells were 33% larger in volume than diploid cells. Since the whole process was fraught with risks and problems, other ways were sought.
Differences in growth between a triploid and diploid oyster after 36 months
Help came from another non-native source, a Chinese geneticist, who emigrated to Seattle in 1985 to pursue postgraduate work, Ximing Guo, and he wanted to go a step further and create a tetraploid oyster (with four sets of chromosomes) which if breeded with a natural diploid would then produce a “natural” triploid, thus avoiding the use of any toxic and cancerous chemical. The problem was that the diploid egg normally was too small to hold two extra sets of chromosomes and all his attempts ended in failure. Meanwhile, Standish Allen had relocalised back to the East coast and gained his first full-time academic post at Rutgers University and its Haskins Shellfish Research Laboratory in 1989. Within a few years, he managed to persuade Guo to join him there and the two started working together on the specific problem of creating a fertile triploid with large enough eggs, although from the outset triploid oysters were supposed to be completely sterile and unable to develop gametes. However, it was occasionally observed that such fertile triploids did exist. So once these triploid oysters and their large eggs were identified, Guo and Allen still resorted to cytochalasin B to ensure that the triploid eggs could be manipulated during meiosis I to accommodate another set of chromosomes from male diploids and then grow into oyster spat. It was found that it was absolutely necessary to monitor the timing of biological indicators in the actual meiotic events in the individual triploid female eggs rather than to follow more general criteria, if tetraploids were to be bred successfully, because of greater variability and asynchrony of triploid eggs than in diploid equivalents. Even then the average success rate after eight days was about 12% (though others have reported much lower figures), and the vast majority of the fertilised eggs were deformed aneuploids. Other critical parameters were salinity and temperature levels and the length of time spent by the eggs immersed in seawater. According to one paper written by these two scientists and two Chinese colleagues[3], the major cause for the formation of tetraploids was a mechanism during a crucial stage of meiosis II, called united bipolar segregation, when the homologous chromosomes are segregated into different cells. It is quite an ironic quirk of nature that the supply of sterile oysters depends on those very same oysters not being sterile at all!

The production of natural triploid zygotes using tetraploid males and diploid females
In 1993, the new tetraploid oyster was created in the laboratory by Guo and Allen: this was the second time Allen had invented an artificial oyster, but now he wasn’t going to miss out on creating a patent for his work. When the supply of tetraploid oysters could be regularly guaranteed, they could be used, more often than not the male species, on a large scale to breed with female diploids so as produce “natural” triploid offsprings to be used for cultivation. These “natural” triploids were after only 9 months of growth as much as 50% larger than normal diploid oysters, which satisfied both the scientists and cultivators alike, and even a third larger than induced triploids. Because of the growing dependency of the oyster industry on hatcheries for supplying oyster seed of Pacific oysters, Crassostrea gigas, there has been a rapid response from both growers and hatcheries to develop the techniques of tri- and tetraploidy, especially the West Coast of North America. Now most of the oyster seed supplied by commercial hatcheries for cultivation there are triploids, produced with the various methods described, although batches produced with older methods often may contain diploid oysters.
| United States Patent |
5,824,841 |
| Guo , et al. |
October 20, 1998 |
Tetraploid shellfish
Abstract
Provided by this invention are novel tetraploid mollusks, including oysters, scallops, clams, mussels and abalone. Also, provided are a method for producing the tetraploid mollusks and a method for producing triploid mollusks by mating the novel tetraploid mollusks with diploid mollusks.
| Inventors: | Guo; Ximing (Glassboro, NJ), Allen, Jr.; Standish K. (Mauricetown, NJ) |
| Assignee: | Rutgers, The State University of New Jersey (New Brunswick, NJ) |
| Appl. No.: | 08/895,077 |
| Filed: | July 16, 1997 |
The patent (United States Patent 5824841) was accordingly granted in 1998 to both Guo and Allen. They went on to set up a special start-up company for the creation of tetraploid molluscs with Rutgers University, 4Cs Breeding Technologies, Inc, which supplies its patented tetraploid oysters to licensed hatcheries wanting to breed 100% guaranteed triploids for cultivation.
So now this is the most common way of producing oyster triploid seed in hatcheries for the oyster cultivation, and this dependency on tetraploid technology has been growing by the year, especially in North America. Allen has continued to work on producing disease-resistant strains of tetraploids and it is easy to see how the research conducted by him and others, for instance, now at the Aquaculture Genetics and Breeding Technology Center within the Virginia Institute of Marine Sciences, on chromosome set manipulation will eventually lead, if not already, to genetic selection, to the development of specific strains of triploid oysters which not only grow faster and bigger, but will also have particular shell characteristics and be able to resist viruses, parasites and pollutants and no doubt even in due course – to the area of transgenics and genetic modification where DNA material from another species is introduced. In addition, there are concerns about the long-term risks over generations of using a mycotoxin, like cytochalasin B, in the creation of first-generation tetraploids, as very little is known about such effects.
Oysters have always been considered, like many other shellfish, as one of the last natural products and have often been marketed as such. If they gradually lose not only this status and also reputation, there may be consequences for their consumption. Fortunately there are stocks of wild oysters still being cultivated and even seed from these stocks, which is sold to other growers and hopefully this will continue and be preserved.
France is another country which has taken on board the benefits of growing triploids, known there as l’huître des quatre saisons – the oyster for the four seasons. Ever since 1997 when IFREMER – a State research institute for marine exploitation – purchased tetraploid oysters to breed, many cultivators have been enthusiastic about buying oyster seed from its hatcheries, which became commercially available 2000. However, ethical controversies still arise about their place and effects in the biological diversity of marine ecosystems and also among consumers who are sceptical to the product.
However, on the other hand, science and man are doing all they can to eclipse nature, but nature will have the last say or laugh whatever and man will always be playing a desperate catch-up game in which the rules are surreptitiously altered and which will probably lead us into an irreversible cataclysm. Already it is estimated that 85% of all native oyster reefs have been made extinct globally, and in many areas the loss is more than 99%[4]. But it is not just the reefs that have disappeared but probably more importantly entire marine ecosystems that the oysters basically provide: such services as water filtration, food and habitat for other species and coastal stabilisation and defence. If sustainability in oyster fishing is to be achieved, reef conservation and management need to be strictly enforced, including the establishment of protected areas and the ban of destructive harvesting practices. A concerted and joint effort from various stakeholders, such as fishermen, aquaculture companies, public agencies, environmental and conservation groups and other NGOs, is absolutely necessary if a long-term rebuilding of oyster reefs and sustainable harvests is to be achieved, rather than the short-sighted goals of put-and-take fishing that has often happened. But all this goes against the grain of the ways and shifts of a life of autonomy that have marked fishermen, watermen and sea-faring communities for centuries; they now also have had to resist being overwhelmed by urbanisation, gentrification and industrialisation. And they have seen the source of their livelihood invaded and taken over by conglomerates and with their backs against the wall have become all the possessive about their marine territory, possibly as a last desperate measure to safeguard its dwindling riches. In a way, who can blame them? Rather, it has been the inevitable spread of urbanised life in all its avatars that has killed the oyster beds, the frenzied demand and over-consumption, disease, pollution and acidification – in a couple of words, modern civilisation. So it is now down to those most exemplary carriers of the latter, the scientists, to come up with laboratory solutions that will repair and restore the depleted oyster banks that once filled our coastal waters.
[1] Villeneuve, A.M. & K. J. Hillers: Whence Meiosis? Cell, 106 (2001), 647-650.
[2] Stanley, J.G., S. K. Allen and H. Hidu: Polyploidy induced in the American Oyster, Crassostrea Virginica, with Cytochalasin B. Aquaculture, 23 (1981), 1-10.
[3] Que, H. et al: Chromosome segregation in fertilised eggs from triploid Pacific oysters. Crassostrea gigas (Thunberg), following inhibition of polar body 1. Biological Bulletin, 193 (1997), 14-19.
[4] Beck, M.W. et al:_Oyster Reefs at risk and recommendations for conservation, restoration and management. Bioscience, 61 (2011), 107-116.


http://en.wikipedia.org/wiki/The_Swarm_(novel)
While the story unfolds the novel touches various topics, including the destruction and poisoning of the maritime ecosystems on earth, the importance of the sea for humanity and the coexistence of different species. The book also remarks on the human inability to thoroughly understand “alien” life; it speculates on the philosophical and religious consequence that the discovery of another sentient species on earth may have. (from wikipedia)
Like an inside the ice huge oyster..https://www.facebook.com/photo.php?fbid=393005457383911&set=a.210721328945659.58322.120085081342618&type=1&theater
Great read! In my experience, most Californian oyster farms are against using trips, and I assume tetras (my first time hearing of them), because of the scientifcally modified stigma they carry. Do you know of any specific farms in the U.S. primarily producing trips? I’d be curious to see the differences first hand, and it’s not exactly like a trip is denoted on the oyster tags.
Thanks! I believe Taylors Shellfish are based in CA and use the tetraploid technology. 4Cs Breeding Technologies have a website and they are mentioned there, but I guess far more buy seed from licensed hatcheries than are prepared to admit openly. That is why more transparency and information about cultivation methods should be provided to the consumer.
“more transparency” INDEED is needed! I live by Tomales Bay and have discovered definite evidence that several (perhaps all) oyster growers – including Drakes Bay Oyster Co. – have used triploids.
The multimillion dollar Pacific oyster industry is oldest and strongest in Washington state – where Taylor Shellfish and the PCSGA (Pacific Coast Shellfish Growers Association) are based.
Since at least 2001 their intensive marketing of genetically engineered shellfish (Pacific oysters, manilla clams, scallops, mussels, geoducks, …?) has successfully pressured growers into deceiving themselves and their customers about the truth – that they are propagating patented organisms whose artificial chromosomal aberrations pose poorly understood and uncontrollable risks to the health of humans and the environment. ‘Mortality events’ and shellfish poisonings from HABS (Harmful Algal Blooms) are increasing in CA and around the world, as is certainty that Pacific oysters become even more destructively invasive in its ‘frankenshell’ form. Determined denial of the truth is more fun and profitable – at least until …? Eat, drink, and be merry, Right? Lycka till.
Thanks Victoria! I wish there were more consumers and growers like you with the same concern and outlook regarding the way we are treating nature and the environment and really ourselves. But the devil/god of consumption has taken over as well as denial. At least we have more resources and instruments now to try and reach out with pertinent and transparent information. But will people listen, see and speak out? And that’s the problem, like those three monkeys….
Thank you, Nigel, that was an incredibly helpful overview! I was a little confused when you used the word “fish” when discussing the first hatcheries–I presume you were really just talking about mollusks?
Due to providing habitat and filtration of the water, the fact that they don’t outcrowd native Olympia oysters (mankind did a nice enough job depleting those stocks on its own), and their presently endemic nature, I wouldn’t be too concerned about Pacific oysters invading the West. In particular, since triploids and tetraploids have such low fertility rates, even if the coast is strewn with them on cultivated oyster farms, if the growers stop putting them out there the polyploid population will not expand as quickly as the diploid population.
I’m of the understanding that most concerns with GMOs are that there are chromosomes added to the organism that didn’t originate from the organism itself, which is not the case with the extra chromosome. Is this not the case? It seems to me about the same as seedless grapes, and nobody’s causing a ruckus over there.
And a small detail for you, Victoria–there aren’t currently commercial polyploid geoducks. Geoduck breeding has in fact been pretty well managed to maintain genetic diversity and not selectively bred, which is why a few scientists are now starting to develop triploids so that if/when the industry starts selective breeding they can minimize cross-breeding with the wild stocks. Of course not a perfect system due to the incomplete sterility, but better than nothing and I doubt we’ll be able to prevent selective breeding in response to the growing demand for geoducks.
Hi Millie!
And thanks for your nice comments!
The problem with triploids in the wild is that a tiny percentage are fertile and can spawn but their offsprings may be deformed as some will be aneuploid (with an abnormal number of chromosomes). Most of them die young but some survive and deformities may be inherited with the population.
Your other point about GMOs – triploids are not genetically modified as no other genetic material from another species is transferred, but I believe that science will want to try its hand at that some day, all under the guise of safeguarding a “healthy” oyster population for aquaculture, probably to be better at withstanding certain diseases. Within 20 years or so, I suspect. I know a lot of oyster farmers see triploids as a godsend but I see it also as the thin edge of science’s (and industry’) wedge to “take control” of the market. But on the other hand, it may help also to safeguard the wild population – hopefully – but 150 years of oyster farming tell a another story. When have wild stocks of fish been successfully protected?
By the way, the first hatcheries I mentioned from the 19th century were for freshwater fish.
Thanks again for your interest, much appreciated. Where are you from?
Cheers
Nigel
Ah, but the deformities arising from polyploidy or aneuploidy are not genetic deformities, that is there is not a gene coding them to pass on to descendents. Instead, those deformities are a result of the cells having a hard time splitting up chromosome numbers evenly. I don’t see how that would be a threat to wild or naturalized oysters. Sure, you could say that polyploid sperm are competing with diploid sperm to fertilize diploid eggs, but polyploid sperm is pretty wimpy and being broadcast spawners there’s going to be a tremendous amount of eggs that just don’t make it.
Polyploidy as a “gateway drug” to genetic modification–sure, that can happen. But like any human decision that can lead to another decision, we need commonsense and not fear tactics to decide where to draw that line. I think it’s worthy to keep it under discussion, but “give them an inch and next they’ll ask to take a mile so let’s not give them anything to start with” isn’t something we should base our policies on.
I’m not under the impression that there have really been 150 years of oyster farming to tell a tale of inability to safeguard wild population. Like you mentioned in your post above, wild stocks aren’t sufficient to maintain demand, so we turn to farming. How long has farming really been intensive? Surely not 150 years, more like the last 30-40 and it seems our whole society is coming around to the outlook that we can’t just keep throwing waste into the waters, which is a far larger threat to oysters than oyster farms. Just don’t get me started on CO2 and ocean acidification, holy crap we’re in trouble on that one…
Hi again!
Concerning this argument for and against the use of triploids where there are wild stocks of oysters, I found this article rather pertinent in Hereditary, 2004, Chromosome inheritance in triploid Pacific oyster Crassostrea gigas Thunberg:
“Since triploids have greatly reduced reproductive potential compared with diploid stocks, the use of triploids in aquaculture can still reduce ‘genetic pollution’ of wild populations by selected strains. On the other hands, the production of aneuploid progeny is unwanted. When large populations of triploids are deployed for aquaculture production, they could affect chromosome number of wild populations”.
I’ve seen other articles where similar doubts have been raised, and I agree there are lots of for’s and against’s
And are you sure that the nucleotide sequences in genes are not disturbed in aneuploidy? I’ll have to check it out more.
All I’m trying to say is that we are treading along a thin balancing line and we need to be aware of ALL the pro’s and con’s as far as possible.
Farming oysters started off in France around 1860 when the idea of artificial breeding was first mooted. The wild population was used but succumbed to diseases, not always caused by farming techniques, obviously, but the latter were not entirely innocent either.
I share your hope that society is becoming more environmentally aware but we are also causing damage on a far higher scale than ever before. I do not think we can play God with nature in the long run. Civilised man is the paradox! Your point about all the waste in our waters, ocean acidification etc is another embarrassing subject. Again let’s have more transparency and discussion.
Try this links
and for Dendropoma petraeum at Egadi
http://www.ampisoleegadi.it/percorsi.html
Ciao Fabio! Thank you so much for the links which I will study when I get home.
It was great to bump into you in the restaurant!
Maybe you can tell me if oysters are cultivated in Italy or even better if they are any wild stocks at all?
I am writing a book about oysters and have written over 100,000 words. Have tried also to write about Sr Orata and his
oysters in Baiae about 80BC.
Yours
Nigel
Millie’s appeal that may be well-intentioned, but it dismisses sensible concerns, with unwarranted prejudice.
Precautionary resistance to the cascade of hazards presented by shellfish aquaculture innovations IS commonsense. Compromise is reckless. Conclusive, conflict-independent research has failed to demonstrate that industrial-scale propagation of genetic abberations is benign to tideland resources and coastal communities. Actually, Millie’s closing distraction over is an additional valid objection: shellfish aquaculture becomes ever-more unsustainable as shell-calicifcation fails and Harmful Algal Blooms (HABS) increase in toxicity. Consumers, and many honest aquaculturists, have been lured into trusting industry propaganda, which poses shellfish as beneficial and sustainable alternative to land-based protein. Millie’s comments provoke my concern because she:
(1) ‘argues from ignorance’
(2) reflects imprudent optimism based in error, ignorance, &/or low standards of care
(3) mimic stock shellfish industry propaganda
Specifically, the keystone quip [aka ‘seedless fruit fallacy’] is a favorite bait-and-switch-trick of the shellfish industry. It pretends that, since {polyploidism in plants occurs naturally and is easily induced in laboratories}, then it must be fine for animals. Polyploidism in animals is profoundly maladaptive, and vanishingly rare. In humans, Spina Bifida and Down’s Syndrome are two well-known results of triploidism: the former of the whole genome, the latter of one particular chromosome.
If geoduck triploids are not yet in commercial production, it is due to the fragile success of sane regulations, which are constantly under assault via PR, lobbyists, and new-media moles. Nevertheless, the industry is extremely destructive [ see: http://protectourshoreline.org/slideshow/POS_ShellfishAquacultureConcerns.pdf ] and research is progressing. Vadopalas, B. and J.P. Davis, 2004, “… evaluated the suitability of cytochalasin B (CB) and 6-dimethylaminopurine (6-DMAP) for triploid induction in geoducks. … found optimal triploid induction (92%) and suitable survivorship (30%) resulted from a 600 µM 6-DMAP treatment.” (Straus et al., 2008) [p.39] “the efficacy of triploidy in conferring sterility in geoducks and the permanence of the triploid state must be verified … any avoidance of genetic risk via harvest management may be counteracted by the increased probability of individual fertilization success among cultured geoducks due to high culture densities.”
According to Washington SeaGrant: Geoducks are long-lived animals, capable of surviving in the wild for 150 years or more. They are broadcast spawners and a female may produce about five billion eggs in its lifetime. … [Puget] Sound’s geoduck population is currently estimated at 674 million pounds, of which approximately 163 million pounds are available for commercial harvest. … Washington’s geoduck farms are presently producing about 875,000 pounds of the clams per year. That compares to about four million pounds of wild geoducks harvested from submerged tidelands each year. Totaled, the two figures account for nearly half the world’s supply of geoduck meat. … Commercial harvesting of geoducks from the wild began in the early 1970s and continues to this day. Until fairly recently, clams in this fishery were gathered from the wild, solely by scuba divers equipped with high-pressure water hoses to blast the deep-burrowing clams from the subtidal sediments. Geoducks imported to Asia can fetch prices of up to $30 per pound, fueling a market currently estimated at around $80 million annually in Washington and British Columbia.
… The State Legislature has identified an assortment of topics that warrant further scientific scrutiny, including possible impacts of the use of sterile triploid geoducks, similar to the so-called “sexless” oysters created in the mid-1980s by researchers at the University of Washington. Other research efforts could focus on geoduck parasites and diseases, impacts from geoduck harvesting, and the extent to which farmed geoducks alter the ecological characteristics of overlying waters while the tracts are submerged at low tide. [ http://www.wsg.washington.edu/communications/seastar/stories/s_07.html ]
Sorry for the info-blitz, I agree with Nigel could educate us all. It should not be misdirected toward ill-informed consensus. Thank you, Nigel, for your rare and lovely forum.
Sorry, my quotes of Millies’ comment did not appear:
(1) ‘argues from ignorance’
“I don’t see how that would be a threat to wild or naturalized oysters”
“I’m not under the impression that there have really been 150 years of oyster farming to tell a tale of inability to safeguard wild population”
(2) reflects imprudent optimism based in error, ignorance, &/or low standards of care
“I wouldn’t be too concerned about Pacific oysters invading the West”
“there’s going to be a tremendous amount of eggs that just don’t make it”
“not a perfect system due to the incomplete sterility, but better than nothing”
“wild stocks aren’t sufficient to maintain demand, so we turn to farming”
“throwing waste into the waters … is a far larger threat to oysters than oyster farms”
[Updated post with all quotes visible]
Millie’s appeal that “we need commonsense and not fear tactics” may be well-intentioned, but it dismisses sensible concerns, with unwarranted prejudice.
Precautionary resistance to the cascade of hazards presented by shellfish aquaculture innovations IS commonsense. Compromise is reckless. Conclusive, conflict-independent research has failed to demonstrate that industrial-scale propagation of genetic abberations is benign to tideland resources and coastal communities. Actually, Millie’s closing distraction over “CO2 and ocean acidification” is an additional valid objection: shellfish aquaculture becomes ever-more unsustainable as shell-calicifcation fails and Harmful Algal Blooms (HABS) increase in toxicity. Consumers, and many honest aquaculturists, have been lured into trusting industry propaganda, which poses shellfish as beneficial and sustainable alternative to land-based protein. Millie’s comments provoke my concern because she:
(1) ‘argues from ignorance’
“I don’t see how that would be a threat to wild or naturalized oysters”
“I’m not under the impression that there have really been 150 years of oyster farming to tell a tale of inability to safeguard wild population”
(2) reflects imprudent optimism based in error, ignorance, &/or low standards of care
“I wouldn’t be too concerned about Pacific oysters invading the West”
“there’s going to be a tremendous amount of eggs that just don’t make it”
“not a perfect system due to the incomplete sterility, but better than nothing”
“wild stocks aren’t sufficient to maintain demand, so we turn to farming”
“throwing waste into the waters … is a far larger threat to oysters than oyster farms”
(3) mimic stock shellfish industry propaganda
Specifically, the keystone quip “It seems to me about the same as seedless grapes” [aka ‘seedless fruit fallacy’] is a favorite bait-and-switch-trick of the shellfish industry. It pretends that, since {polyploidism in plants occurs naturally and is easily induced in laboratories}, then it must be fine for animals. Polyploidism in animals is profoundly maladaptive, and vanishingly rare. In humans, Spina Bifida and Down’s Syndrome are two well-known results of triploidism: the former of the whole genome, the latter of one particular chromosome.
If geoduck triploids are not yet in commercial production, it is due to the fragile success of sane regulations, which are constantly under assault via PR, lobbyists, and new-media moles. Nevertheless, the industry is extremely destructive [ see: http://protectourshoreline.org/slideshow/POS_ShellfishAquacultureConcerns.pdf ] and research is progressing. Vadopalas, B. and J.P. Davis, 2004, “… evaluated the suitability of cytochalasin B (CB) and 6-dimethylaminopurine (6-DMAP) for triploid induction in geoducks. … found optimal triploid induction (92%) and suitable survivorship (30%) resulted from a 600 µM 6-DMAP treatment.” (Straus et al., 2008) [p.39] “the efficacy of triploidy in conferring sterility in geoducks and the permanence of the triploid state must be verified … any avoidance of genetic risk via harvest management may be counteracted by the increased probability of individual fertilization success among cultured geoducks due to high culture densities.”
According to Washington SeaGrant: Geoducks are long-lived animals, capable of surviving in the wild for 150 years or more. They are broadcast spawners and a female may produce about five billion eggs in its lifetime. … [Puget] Sound’s geoduck population is currently estimated at 674 million pounds, of which approximately 163 million pounds are available for commercial harvest. … Washington’s geoduck farms are presently producing about 875,000 pounds of the clams per year. That compares to about four million pounds of wild geoducks harvested from submerged tidelands each year. Totaled, the two figures account for nearly half the world’s supply of geoduck meat. … Commercial harvesting of geoducks from the wild began in the early 1970s and continues to this day. Until fairly recently, clams in this fishery were gathered from the wild, solely by scuba divers equipped with high-pressure water hoses to blast the deep-burrowing clams from the subtidal sediments. Geoducks imported to Asia can fetch prices of up to $30 per pound, fueling a market currently estimated at around $80 million annually in Washington and British Columbia.
… The State Legislature has identified an assortment of topics that warrant further scientific scrutiny, including possible impacts of the use of sterile triploid geoducks, similar to the so-called “sexless” oysters created in the mid-1980s by researchers at the University of Washington. Other research efforts could focus on geoduck parasites and diseases, impacts from geoduck harvesting, and the extent to which farmed geoducks alter the ecological characteristics of overlying waters while the tracts are submerged at low tide. [ http://www.wsg.washington.edu/communications/seastar/stories/s_07.html ]
Sorry for the info-blitz, I agree with Nigel “more transparency and discussion” could educate us all. It should not be misdirected toward ill-informed consensus.
Thank you, Nigel, for your rare and lovely forum.
Thanks Victoria for all your amazing input!It’s great when people like you really show how they care for the only environment we have and expose the hypocrisy and double-talk that goes on in the name of science and advancement. And look at all those watered-down compromises and policies that all these international conferences have managed to produce, while Rome burns! I don’t think we can avoid fighting for sustainability and organic thinking in land and sea farming. That is the bottom line, really! But how to curb hubris, greed, short-sightedness and envy? Great stuff!
If the choice is left to the shellfish industry than naturally the majority will come down on the side of economics.That’s why they are doing it ,to make money.So enviromental concerns or issues about gmo foods will take a back seat.Most don’t care as long as they can increase profits that is what matters and that’s understandable.Unfortunately the long term consequences have not been taken into consideration at any level.This gene modification is taking place without due consultation with the broader community.
Hi Graeme and thanks for your thoughts. Oyster farming, especially of the wild banks, and as with any natural resource, has been blighted by “smash-and-grab” economics. Look at the problems of the Chesapeake Bay and the coasts of France. Concerns about the environment are held by a minority, unfortunately, and sometimes science plays the sorcerer’s apprentice in all this, and I’m inclined to think that the triploid oyster may be a bit of a Trojan horse in the long term. Even though the triploid is not genetically modified, I think one of the next steps in the near future will undoubtedly be the introduction of a GM oyster.
Inspired by Graeme’s input, I did a quick Google search and found a few citations illuminating some emergent concerns of transgenic oysters (and other living beings). I am particularly disturbed by unrecognized threats from introducing Mammalian Growth Hormone genetics into seafood. One concern is the potential for these to present novel ‘endocrine disruptor’ effects, which research now is revealing to seriously impact reproductive health, auto-immune function, and life-threatening cell-growth abnormalities including cancer. Sorry to be such a bummer.
I find it curious that none of the works readily accessible is very recent – might this indicate ‘nothing new’ or ‘nothing we want you to know about’? Surely some deeper effort could improve on this little list:
This review, which also covers shellfish and other aquatic species, includes a glossary of key terms and a basic introduction to technical details of this field.
“Evaluating the transgenic animals and food products,” by GS Harper, in
Transgenic Livestock Review CSIRO 12 Dec2003, available for download through http://www.foodstandards.gov.au
Also from 2003 is this report on a SeaGrant supported effort to create transgenic oysters “using retroviral infection.”
http://escholarship.org/uc/item/4vt1x4qp#page-2
For insight on transgenic vs. traditional hybrids see, also from 2003, “Patenting and Transgenic Organisms: A Philosphical Exploration,” by K.Lee
Click to access Lee(ce).pdf
In 1999 a team at LSU reported on their techniques for gene transfer in oyster gametes and embryos – capable of introducing a gene isolated from a jellyfish. The ‘successful’ oysters produce a protein which emits a bright green when exposed to fluorescent light; the transgenic ‘green-glow’ effect was apparent in oyster larvae.
http://text.lsuagcenter.com/en/communications/publications/agmag/Archive/1999/fall/Gene+transfer+in+oysters.htm
This 2001 study, “documents the first successful insertion and expression of foreign DNA in eastern oyster larvae.”
http://link.springer.com/article/10.1007%2Fs10126-001-0002-9#page-2
A 1995 summary of “Transgenic Fish and Aquaculture,” by T.T.Chen, is available at
Click to access adsea94p081-089.pdf
Hi Victoria!
Thank you once more for your very salient comments about the threat of transgenics. Fascinating thoughts! It seems a lot of the research is taking place in countries like China and Brazil (or maybe even in other less transparent countries, like Panama….) where there might well indeed be a need to produce a cow or goat that will produce a certain kind of milk to prevent, for example, diarrhea. But with fish, they are more difficult to control in the sea, although a GMO salmon has, I think, almost been cleared by the American FDA (see here).
But another article last year in Nature raises the question why research in transgenics has tailed off in the USA….interesting, since public funding has fizzled out, but I don’t think that will stop scientists trying to either make a bit on the side or acquiesce to the interests of some unscrupulous businessmen.So that is why we need to keep walking the talk about some very grave environmental concerns before it is too late. I’m sure there’s lots going on well away from the public glare
Very interesting. I’m just beginning to learn about oysters (even though I live in Virginia where oysters are big business). Thank you for clarifying how triploids are engineered. I shared this post on my blog. https://carolynoneal.wordpress.com/
Thanks, Carolyn, for your comment. Will be visiting Virginia next month to see those ersters for myself!
Yes, oysters are not plants, but most who object to GMO are very likely unaware the majority of plant species we consume today have genetic and/or chromosomal manipulations going back many years to thousands of years (as in selective breeding). The main distinction today in the insertion of “foreign” genetic material from other, usually unreleated species.